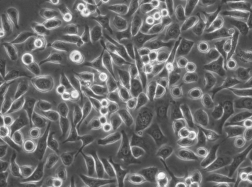
人肺腺癌细胞PC-9
BLUEFBIO™ Product Sheet
|
细胞名称 |
人肺腺癌细胞PC-9 |
|
|
|
货物编码 |
BFN60800699 |
||
|
产品规格 |
T25培养瓶x1 |
1.5ml冻存管x2 |
|
|
细胞数量 |
1x10^6 |
1x10^6 |
|
|
保存温度 |
37℃ |
-198℃ |
|
|
运输方式 |
常温保温运输 |
干冰运输 |
|
|
安全等级 |
1 |
||
|
用途限制 |
仅供科研用途 1类 |
||
|
培养体系 |
DMEM高糖培养基(Hyclone)+10%胎牛血清(Gibco)+1%双抗(Hyclone) |
||
|
培养温度 |
37℃ |
二氧化碳浓度 |
5% |
|
简介 |
人肺腺癌细胞PC-9 于1989年取自一位日本人肺癌组织。 |
||
|
注释 |
Part of: Cancer Cell Line Encyclopedia (CCLE) project. Part of: MD Anderson Cell Lines Project. From: Immuno-Biological Laboratories (IBL); Tokyo; Japan. Omics: Deep exome analysis. Omics: Deep membrane proteome analysis. Omics: Deep phosphoproteome analysis. Omics: Deep RNAseq analysis. Omics: Protein expression by reverse-phase protein arrays. Omics: SNP array analysis. Omics: Transcriptome analysis. |
||
|
STR信息 |
Amelogenin X CSF1PO 11 D2S1338 19,20 D3S1358 16 D5S818 11 D7S820 10,11 D8S1179 11,15 D13S317 8 D16S539 9 D18S51 15 D19S433 13,15.2 D21S11 30 FGA 23 Penta D 13 Penta E 11 TH01 7 TPOX 11 vWA 17 |
||
|
参考文献 |
DOI=10.5795scc.24.451 Tsumuraya M., Nakajima T., Terasaki T., Kodama T., Shimosato Y., Higuchi H., Uei Y. Analysis of LDH isoenzyme patterns in cell lines of the small cell carcinoma (lung) and retinoblastoma. Nihon Rinsho Saibo Gakkai Zasshi 24:451-456(1985)
PubMed=4094096 Lee Y.-C., Saijo N., Sasaki Y., Takahashi H., Sakurai M., Ishihara J., Hoshi A., Chen K.-M., Hamburger A.W. Clonogenic patterns of human pulmonary adenocarcinoma cell lines (PC-9, PC-13 and PC-14) and how they influence the results of test for chemosensitivity to cisplatin in the human tumor clonogenic assay. Jpn. J. Clin. Oncol. 15:637-644(1985)
PubMed=1847845; DOI=10.1007/BF00685110 Sasaki Y., Shinkai T., Eguchi K., Tamura T., Ohe Y., Ohmori T., Saijo N. Prediction of the antitumor activity of new platinum analogs based on their ex vivo pharmacodynamics as determined by bioassay. Cancer Chemother. Pharmacol. 27:263-270(1991)
PubMed=1737336 Yoshino I., Yano T., Murata M., Ishida T., Sugimachi K., Kimura G., Nomoto K. Tumor-reactive T-cells accumulate in lung cancer tissues but fail to respond due to tumor cell-derived factor. Cancer Res. 52:775-781(1992)
PubMed=9023415; DOI=10.1006/cimm.1996.1062 Seki N., Hoshino T., Kikuchi M., Hayashi A., Itoh K. HLA-A locus-restricted and tumor-specific CTLs in tumor-infiltrating lymphocytes of patients with non-small cell lung cancer. Cell. Immunol. 175:101-110(1997)
PubMed=9178645; DOI=10.1006/cimm.1997.1108 Nakao M., Sata M., Saitsu H., Yutani S., Kawamoto M., Kojiro M., Itoh K. CD4+ hepatic cancer-specific cytotoxic T lymphocytes in patients with hepatocellular carcinoma. Cell. Immunol. 177:176-181(1997)
PubMed=11005564; DOI=10.1038/sj.neo.7900094 Kohno T., Sato T., Takakura S., Takei K., Inoue K., Nishioka M., Yokota J. Mutation and expression of the DCC gene in human lung cancer. Neoplasia 2:300-305(2000)
PubMed=11078804; DOI=10.3892/ijo.17.6.1187 Takenaka K., Shibuya M., Takeda Y., Hibino S., Gemma A., Ono Y., Kudoh S. Altered expression and function of beta1 integrins in a highly metastatic human lung adenocarcinoma cell line. Int. J. Oncol. 17:1187-1194(2000)
PubMed=16105816; DOI=10.1158/0008-5472.CAN-05-0331 Nagai Y., Miyazawa H., Huqun X., Tanaka T., Udagawa K., Kato M., Fukuyama S., Yokote A., Kobayashi K., Kanazawa M., Hagiwara K. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276-7282(2005)
PubMed=17332333; DOI=10.1158/0008-5472.CAN-06-3339 Okabe T., Okamoto I., Tamura K., Terashima M., Yoshida T., Satoh T., Takada M., Fukuoka M., Nakagawa K. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 67:2046-2053(2007)
PubMed=17511773; DOI=10.1111/j.1349-7006.2007.00507.x Fukuyama T., Ichiki Y., Yamada S., Shigematsu Y., Baba T., Nagata Y., Mizukami M., Sugaya M., Takenoyama M., Hanagiri T., Sugio K., Yasumoto K. Cytokine production of lung cancer cell lines: correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci. 98:1048-1054(2007)
PubMed=19472407; DOI=10.1002/humu.21028 Blanco R., Iwakawa R., Tang M., Kohno T., Angulo B., Pio R., Montuenga L.M., Minna J.D., Yokota J., Sanchez-Cespedes M. A gene-alteration profile of human lung cancer cell lines. Hum. Mutat. 30:1199-1206(2009)
PubMed=19799608; DOI=10.1111/j.1349-7006.2009.01351.x Kuroda K., Takenoyama M., Baba T., Shigematsu Y., Shiota H., Ichiki Y., Yasuda M., Uramoto H., Hanagiri T., Yasumoto K. Identification of ribosomal protein L19 as a novel tumor antigen recognized by autologous cytotoxic T lymphocytes in lung adenocarcinoma. Cancer Sci. 101:46-53(2010)
PubMed=20215515; DOI=10.1158/0008-5472.CAN-09-3458 Rothenberg S.M., Mohapatra G., Rivera M.N., Winokur D., Greninger P., Nitta M., Sadow P.M., Sooriyakumar G., Brannigan B.W., Ulman M.J., Perera R.M., Wang R., Tam A., Ma X.-J., Erlander M., Sgroi D.C., Rocco J.W., Lingen M.W., Cohen E.E.W., Louis D.N., Settleman J., Haber D.A. A genome-wide screen for microdeletions reveals disruption of polarity complex genes in diverse human cancers. Cancer Res. 70:2158-2164(2010)
PubMed=22313637; DOI=10.4161/cbt.19238 Takata M., Chikumi H., Miyake N., Adachi K., Kanamori Y., Yamasaki A., Igishi T., Burioka N., Nanba E., Shimizu E. Lack of AKT activation in lung cancer cells with EGFR mutation is a novel marker of cetuximab sensitivity. Cancer Biol. Ther. 13:369-378(2012)
PubMed=23733853; DOI=10.1101/gr.152322.112 Jia P., Jin H., Meador C.B., Xia J., Ohashi K., Liu L., Pirazzoli V., Dahlman K.B., Politi K., Michor F., Zhao Z., Pao W. Next-generation sequencing of paired tyrosine kinase inhibitor-sensitive and -resistant EGFR mutant lung cancer cell lines identifies spectrum of DNA changes associated with drug resistance. Genome Res. 23:1434-1445(2013)
PubMed=26202522; DOI=10.1021/acs.jproteome.5b00477 Kitata R.B., Dimayacyac-Esleta B.R., Choong W.-K., Tsai C.-F., Lin T.-D., Tsou C.-C., Weng S.-H., Chen Y.-J., Yang P.-C., Arco S.D., Nesvizhskii A.I., Sung T.-Y., Chen Y.-J. Mining missing membrane proteins by high-pH reverse-phase stagetip fractionation and multiple reaction monitoring mass spectrometry. J. Proteome Res. 14:3658-3669(2015)
PubMed=26554430; DOI=10.1021/acs.analchem.5b03639 Dimayacyac-Esleta B.R., Tsai C.-F., Kitata R.B., Lin P.-Y., Choong W.-K., Lin T.-D., Wang Y.-T., Weng S.-H., Yang P.-C., Arco S.D., Sung T.-Y., Chen Y.-J. Rapid high-pH reverse phase stagetip for sensitive small-scale membrane proteomic profiling. Anal. Chem. 87:12016-12023(2015)
PubMed=28196595; DOI=10.1016/j.ccell.2017.01.005 Li J., Zhao W., Akbani R., Liu W., Ju Z., Ling S., Vellano C.P., Roebuck P., Yu Q., Eterovic A.K., Byers L.A., Davies M.A., Deng W., Gopal Y.N.V., Chen G., von Euw E.M., Slamon D.J., Conklin D., Heymach J.V., Gazdar A.F., Minna J.D., Myers J.N., Lu Y., Mills G.B., Liang H. Characterization of human cancer cell lines by reverse-phase protein arrays. Cancer Cell 31:225-239(2017)
PubMed=28290473; DOI=10.1038ep44021 Wang Y.-T., Pan S.-H., Tsai C.-F., Kuo T.-C., Hsu Y.-L., Yen H.-Y., Choong W.-K., Wu H.-Y., Liao Y.-C., Hong T.-M., Sung T.-Y., Yang P.-C., Chen Y.-J. Phosphoproteomics reveals HMGA1, a CK2 substrate, as a drug-resistant target in non-small cell lung cancer. Sci. Rep. 7:44021-44021(2017)
PubMed=31068700; DOI=10.1038/s41586-019-1186-3 Ghandi M., Huang F.W., Jane-Valbuena J., Kryukov G.V., Lo C.C., McDonald E.R. III, Barretina J., Gelfand E.T., Bielski C.M., Li H., Hu K., Andreev-Drakhlin A.Y., Kim J., Hess J.M., Haas B.J., Aguet F., Weir B.A., Rothberg M.V., Paolella B.R., Lawrence M.S., Akbani R., Lu Y., Tiv H.L., Gokhale P.C., de Weck A., Mansour A.A., Oh C., Shih J., Hadi K., Rosen Y., Bistline J., Venkatesan K., Reddy A., Sonkin D., Liu M., Lehar J., Korn J.M., Porter D.A., Jones M.D., Golji J., Caponigro G., Taylor J.E., Dunning C.M., Creech A.L., Warren A.C., McFarland J.M., Zamanighomi M., Kauffmann A., Stransky N., Imielinski M., Maruvka Y.E., Cherniack A.D., Tsherniak A., Vazquez F., Jaffe J.D., Lane A.A., Weinstock D.M., Johannessen C.M., Morrissey M.P., Stegmeier F., Schlegel R., Hahn W.C., Getz G., Mills G.B., Boehm J.S., Golub T.R., Garraway L.A., Sellers W.R. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569:503-508(2019) |
||
验收细胞注意事项
1、收到人肺腺癌细胞PC-9细胞,请查看瓶子是否有破裂,培养基是否漏出,是否浑浊,如有请尽快联系。
2、收到人肺腺癌细胞PC-9细胞,如包装完好,请在显微镜下观察细胞。,由于运输过程中的问题,细胞培养瓶中的贴壁细胞有可能从瓶壁中脱落下来,显微镜下观察会出现细胞悬浮的情况,出现此状态时,请不要打开细胞培养瓶,应立即将培养瓶置于细胞培养箱里静止 3-5 小时左右,让细胞先稳定下,再于显微镜下观察,此时多数细胞会重新贴附于瓶壁。如细胞仍不能贴壁,请用台盼蓝染色法鉴定细胞活力,如台盼蓝染色证实细胞活力正常请按悬浮细胞的方法处理。
3、收到人肺腺癌细胞PC-9细胞后,请镜下观察细胞,用恰当方式处理细胞。若悬浮的细胞较多,请离心收集细胞,接种到一个新的培养瓶中。弃掉原液,使用新鲜配制的培养基,使用进口胎牛血清。刚接到细胞,若细胞不多时 血清浓度可以加到 15%去培养。若细胞迏到 80%左右 ,血清浓度还是在 10%。
4、收到人肺腺癌细胞PC-9细胞时如无异常情况 ,请在显微镜下观察细胞密度,如为贴壁细胞,未超过80%汇合度时,将培养瓶中培养基吸出,留下 5-10ML 培养基继续培养:超过 80%汇合度时,请按细胞培养条件传代培养。如为悬浮细胞,吸出培养液,1000 转/分钟离心 3 分钟,吸出上清,管底细胞用新鲜培养基悬浮细胞后移回培养瓶。
5、将培养瓶置于 37℃培养箱中培养,盖子微微拧松。吸出的培养基可以保存在灭菌过的瓶子里,存放于 4℃冰箱,以备不时之需。
6、24 小时后,人肺腺癌细胞PC-9细胞形态已恢复并贴满瓶壁,即可传代。(贴壁细胞)将培养瓶里的培养基倒去,加 3-5ml(以能覆盖细胞生长面为准)PBS 或 Hanks’液洗涤后弃去。加 0.5-1ml 0.25%含 EDTA 的胰酶消化,消化时间以具体细胞为准,一般 1-3 分钟,不超过 5 分钟。可以放入37℃培养箱消化。轻轻晃动瓶壁,见细胞脱落下来,加入 3-5ml 培养基终止消化。用移液管轻轻吹打瓶壁上的细胞,使之完全脱落,然后将溶液吸入离心管内离心,1000rpm/5min。弃上清,视细胞数量决定分瓶数,一般一传二,如细胞量多可一传三,有些细胞不易传得过稀,有些生长较快的细胞则可以多传几瓶,以具体细胞和经验为准。(悬浮细胞)用移液管轻轻吹打瓶壁,直接将溶液吸入离心管离心即可。
7、贴壁细胞 ,悬浮细胞。严格无菌操作。换液时,换新的细胞培养瓶和换新鲜的培养液,37℃,5%CO2 培养。
特别提醒: 原瓶中培养基不宜继续使用,请更换新鲜培养基培养。